

Add the valence electrons to the molecular orbital diagram.The 2p y orbitals on each carbon combine to make another 2 pi symmetry orbitals, 90 degrees from the first set. The 2p x orbitals on each atom combine to make 2 pi symmetry orbitals.(C-H bonds)Ĭombine the other 2 C(2sp) orbitals to make a sigma bonding and a sigma antibonding molecular orbital. Combine each H(1s) orbital with a C(2sp) orbital to make a sigma bonding and a sigma antibonding molecular orbital.After hybridization, a 2p x and a 2p y orbital remain on each carbon atom. The carbon atoms in ethyne use 2sp hybrid orbitals to make their sigma bonds.Each carbon atom makes 2 sigma bonds and has no lone pairs of electrons. Each carbon has 4 and each hydrogen 1 for a total of 12 electrons.Įthyne, sp hybridization with two pi bonds Finally, add the valence electrons to the molecular orbital diagram.The stabilization and destabilization in forming a pi bond are much less than for a sigma bond. The stabilization (decrease in energy) in going from the p orbital to pi bonding orbital equals the destabilization (increase in energy) in going from the p orbital to the pi antibonding orbital. These can combine to make a pi bonding and a pi antibonding molecular orbital. There remains a 2p orbital on each carbon.There are no remaining hybrid orbitals.(C-H bonds)Ĭombine the 2 C(2sp 2) orbitals to make a sigma bonding and a sigma antibonding molecular orbital. understand the formation of sigma and pi bonds and know their occurrence and the implication for molecular shapes The concept of mixing atomic orbitals. Combine each H(1s) orbital with a C(2sp 2) orbital to make a sigma bonding and a sigma antibonding molecular orbital.As with borane, make 2sp 2 hybrid orbitals on each carbon from the 2s, 2p x, and 2p y atomic orbitals.Each carbon forms 3 sigma bonds and has no lone pairs.
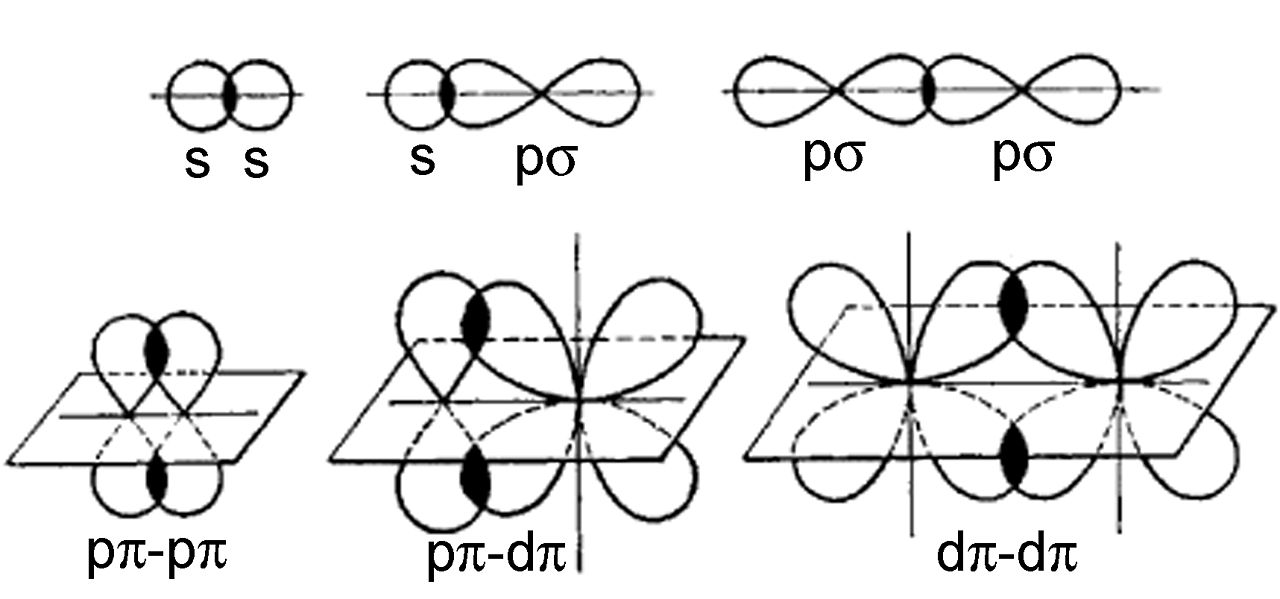
The Lewis structure of the molecule CH 2CH 2 is below.They are not formed from hybrid orbitals. Pi bonds are formed from the overlap of parallel p orbitals on adjacent atoms. If a bond between two atoms is broken when one atom is rotated around the bond axis, that bond is called a pi bond. If two atoms are connected by a sigma bond, rotating one of the atoms around the bond axis doesn't break the bond. Sigma bonds are formed by the overlap of orbitals that are pointing directly towards one another. Hybrid orbitals are constructed from valence atomic orbitals and used to make sigma bonds between atoms.


 0 kommentar(er)
0 kommentar(er)
